Explain the Difference Between Exothermic and Endothermic
The difference between catalysts and enzymes is that enzymes are largely organic in nature and are bio-catalysts while non-enzymatic catalysts can be inorganic compounds. Explain this difference in boiling point in terms of all the intermolecular forces present between molecules of each substance.
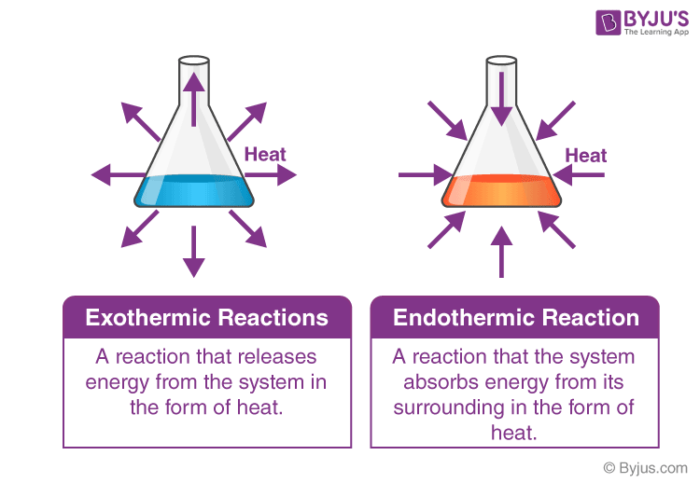
Difference Between Endothermic And Exothermic Reactions Chemistry
Justify your answer in terms of.
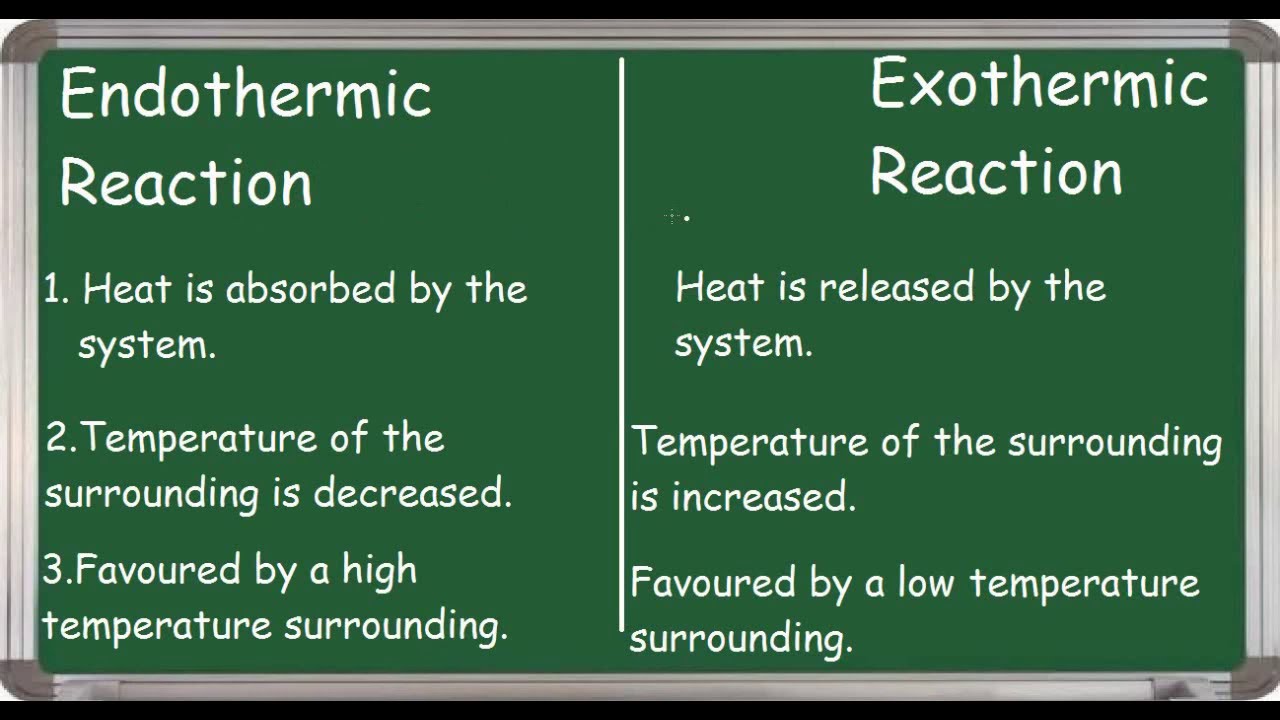
. On this information is the dissolution of urea endothermic or exothermic. General energy diagrams for exothermic and endothermic reactions 2020 Lets Talk Science. Includes kit list and safety instructions.
The classifications are related to endothermic and exothermic reactions except endergonic and exergonic describe what happens with any form of energy while endothermic. In fact all known enzymes are catalysts but not all catalysts are enzymes. Is 332 K whereas the boiling point of BrCl is 278 K.
The exothermic reaction is the opposite of an endothermic reaction. The more interesting quantity is the change of enthalpy the total energy that was exchanged within a system. Endergonic reactions include endothermic reactions.
Design experiments with different reactions concentrations and temperatures. Because heat is released when these new attractive interactions form dissolving most gases in liquids is an exothermic process ΔH soln 0. Explore what makes a reaction happen by colliding atoms and molecules.
Identifying Exothermic Endothermic Reactions. Ionization energy is the amount of energy necessary to remove an electron from an atom. Due to that reason the change in enthalpy is a positive value the change in the enthalpy is the difference between the enthalpies of products and reactants.
Endergonic and exergonic are two types of chemical reactions or processes in thermochemistry or physical chemistryThe names describe what happens to energy during the reaction. Then according to the above equation the ΔG is a positive value. When energy is absorbed in an endothermic reaction the temperature decreases.
Monitor temperature change When energy is released in an exothermic reaction the temperature of the reaction mixture increases. Since new products are formed the entropy of the system is decreased. Illustrate the reaction between glycerol and potassium manganateVII to produce flames and steam in this demonstration.
The ionization energy tends to increase from left to right across the periodic table because of the increase number of protons in the nucleus of the atom. There are two methods for distinguishing between exothermic and endothermic reactions. A spontaneous exothermic reaction.
It tends to decrease down a column of the periodic table because the number of electron shells is larger making each ion further away. When are reactions reversible. Enzymes and catalysts both affect the rate of a reaction.
Conversely adding heat to the solution provides thermal energy that overcomes the attractive forces between the gas and the solvent molecules thereby decreasing the solubility of the gas. It is a state function depending only on the equilibrium state of a system. What affects the rate of a reaction.
In association with Nuffield Foundation. Difference Between Endothermic and Exothermic Reactions. Enthalpy measures the total energy of a thermodynamic system either in the form of heat or volume multiplied by pressure.
The difference between the energy levels of reactants and products is called the enthalpy change ΔH. It is a simplified description of the energy transfer energy is. It releases energy by light or heat to its surrounding.
Neither catalysts nor enzymes are consumed in the reactions they catalyze. You can see from the diagram above that the energy level of the products of an exothermic reaction is lower than the energy level of the reactants. A few examples are neutralization burning a substance reactions of fuels deposition of dry ice respiration solution of sulfuric acid into water and much more.

Difference Between Endothermic And Exothermic Reaction Brainly In

Endothermic Vs Exothermic Reaction Differences Youtube
Difference Between Endothermic And Exothermic Reactions Definition Properties Examples
Comments
Post a Comment